|
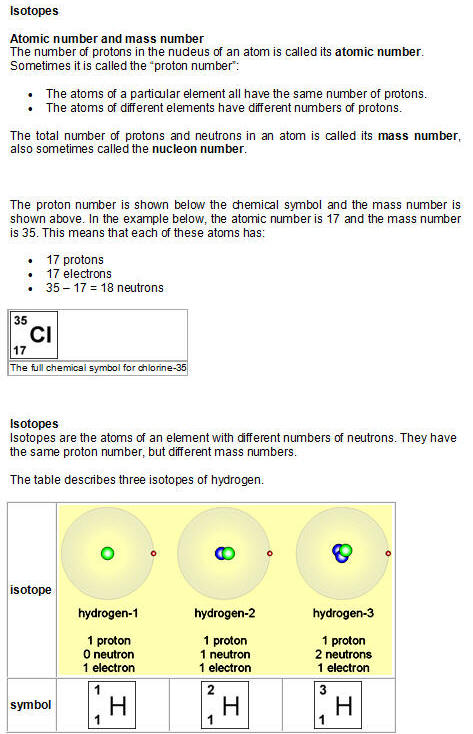
Your teacher will explain what is meant by
the terms atomic number
and mass number, and how
to calculate the number of neutrons
in an atom.
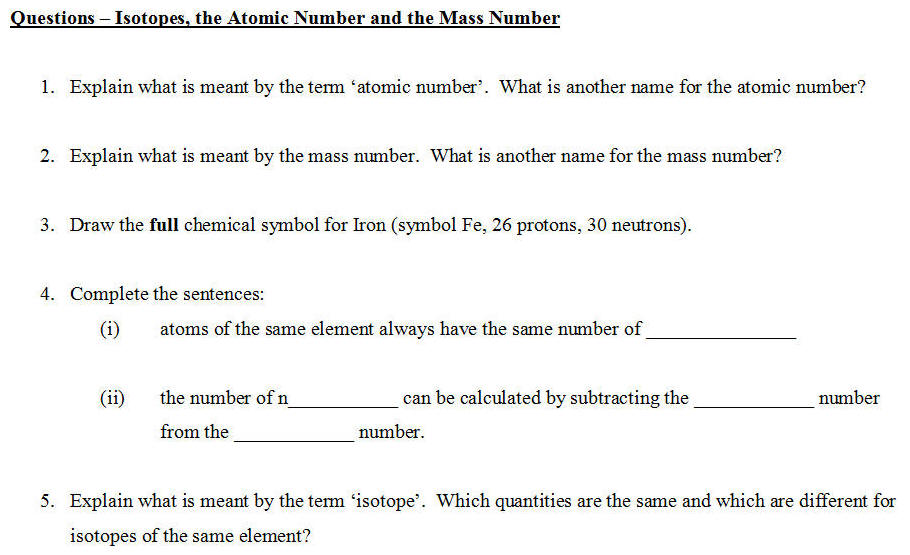
1)
What is meant by atomic number.
What is
another name for the atomic number?
2) Explain what is meant by
mass number.
What is another name for the mass number?
3) Draw the
full chemical symbol for Iron.
(symbol Fe, 26 protons, 30 neutrons)
4)
Fill in the missing words:
Atoms of the same element always have the
same number of ____________. The number of n___________
can be calculated by subtracting the ____________ number from
the ____________ number.
5)
What is meant by the term
isotope.
Which quantities are the same and which are different
for isotopes of the same element?
Click the image above to
download these questions as a word document.
Your teacher
may give you a copy of the information sheet
below, to help you answer the questions.
Click the image above to
download the information sheet.
|

